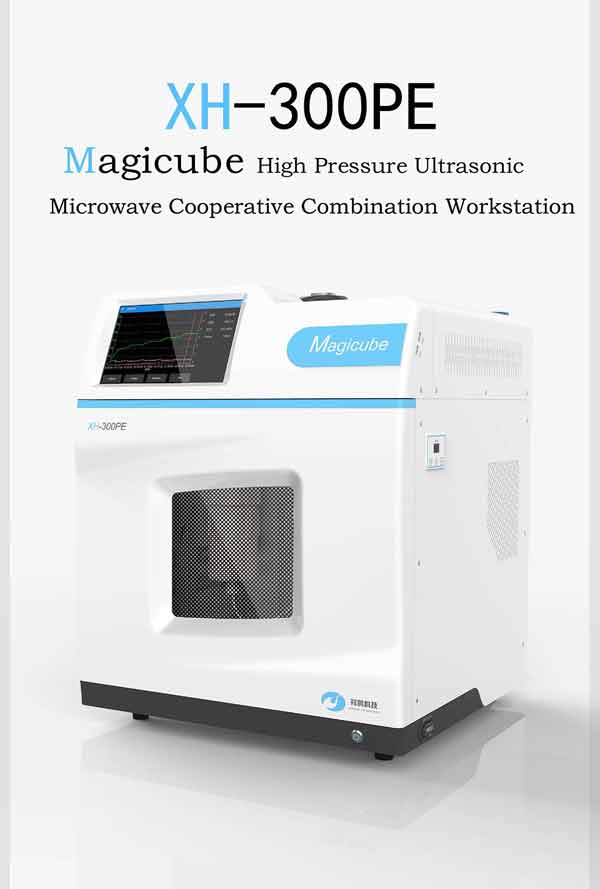
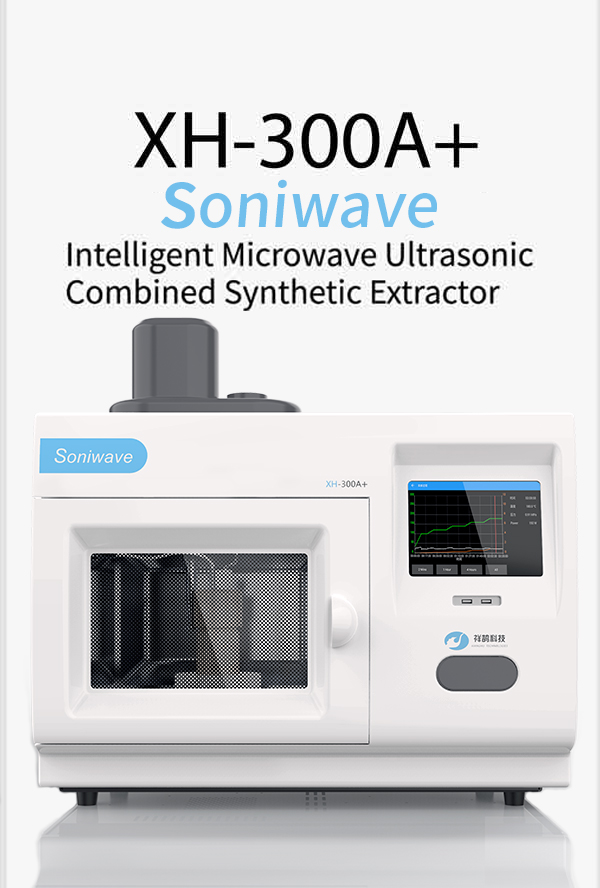
- 路 Microwave
- 路 Atmospheric Pressure Microwave 路 Pressure Microwave 路 Parallel Microwave
- 路 Ultrasonic 路Low Temperature Ultrasound
- 路 Ultraviolet Light
- 路 Microwave Heating 路 Atmospheric Pressure Synthesis 路 Atmospheric Pressure Catalysis 路 Atmospheric Pressure Extraction
- 路 Sample Preparation 路 Microwave Digestion
- 路 Soil Digestion 路 High Pressure Synthesis
- 路 Solid Phase Synthesis
- 路 Organic Synthesis
- 路 Ionic Liquid Synthesis
- 路 Degradation Of Natural Organic Matter
- 路 Natural Product Extraction / Purification
河北祥鹄科学仪器有限公司
300A Study on Luminescent Properties of Eu_3_Doped CaF_Omitted_ and LaF_3 Phosphors Modified by Ligand
This paper was completed by a researcher from the Key Laboratory of Condensed Phase Change and Microstructure of Yili Normal University. The paper discusses the study on the luminescence properties of Eu3 + -doped CaF2 and LaF3 phosphors under ligand modification. It is published in the important journal
Eu3 + doped LaF3 and CaF2 phosphors with special fluorescence properties were successfully synthesized by microwave method. The structure, morphology and luminescence properties of the samples were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM) and photoluminescence spectroscopy (PL). The effects of sodium tartrate and Eu3 + doping on the luminescence properties of synthetic phosphors were discussed. The experimental results show that the addition of sodium tartrate and the doping amount of Eu3 + have a great influence on the fluorescence performance. The change of molar ratio makes Eu3 + change from magnetic dipole transition to electric dipole transition dominated, and then the main peak The position changes from the previous 588 nm to 613 nm. However, it did not cause changes in other peak positions, indicating that Eu3 + entered the lattice of LaF3 and CaF2 without any other changes.

Fig.1/3↑

Fig.2/3↑

Fig.3/3↑
Calcium fluoride and cesium fluoride were used as matrix materials to investigate the effect of the amount of ligand added and the doping amount of activated ion Eu3 + on its fluorescence intensity and luminescence position. The results show that LaF3.. Eu3 + is an ellipsoidal particle; CaF2 .. Eu3 + is a nearly cubic particle. The addition of sodium tartrate and the amount of Eu3 + doping can change the position of Eu3 + in the lattice of LaF3 and CaF 2 , which in turn affects the position of the main peak of the emission peak and the intensity of the emission peak. With LaF3 as the matrix, when the amount of sodium tartrate added was N = 2, the red phosphor with 5D0 →7F2 transition was obtained; when Eu3 + doping amount x = 0. At 6 o'clock, a phosphor with both 5D0 →7F2 transitions and 5D0 →7F1 transitions was obtained; with CaF 2 as the matrix, when the amount of sodium tartrate added was N = 5, when the Eu3 + doping amount was x = 0.4, Eu3 + changes in the position of CaF2, and a red phosphor based on 5D0→ 7F2 transition is obtained.
(1 - x) mmol anhydrous calcium chloride CaCl2, La(NO3) 3· 6H2O were dissolved in 50 mL of deionized water with x mmol of lanthanum lanthanum Eu (NO3) 3 · 6H2O to form solution A, N at room temperature. The mmol of sodium tartrate is dissolved in 100 mL of deionized water to form solution B. A certain amount of sodium fluoroborate NaBF4 is dissolved in 50 mL of deionized water to form solution C. First, the solution A is slowly poured into B and mixed uniformly. Then, the mixed solution of A and B is placed in a microwave ultrasonic combined synthesis/extraction instrument with a power of 600 W. Set 120 ° C, heat to reflux until the solution boiled, then add solution C dropwise in the microwave, continue the reaction for 30 min to obtain a white precipitate, wash 3 times with deionized water, and dry at room temperature to obtain a sample.








































 京ICP备15050585号
京ICP备15050585号

