- 路 Microwave
- 路 Atmospheric Pressure Microwave 路 Pressure Microwave 路 Parallel Microwave
- 路 Ultrasonic 路Low Temperature Ultrasound
- 路 Ultraviolet Light
- 路 Microwave Heating 路 Atmospheric Pressure Synthesis 路 Atmospheric Pressure Catalysis 路 Atmospheric Pressure Extraction
- 路 Sample Preparation 路 Microwave Digestion
- 路 Soil Digestion 路 High Pressure Synthesis
- 路 Solid Phase Synthesis
- 路 Organic Synthesis
- 路 Ionic Liquid Synthesis
- 路 Degradation Of Natural Organic Matter
- 路 Natural Product Extraction / Purification
河北祥鹄科学仪器有限公司
10 Preparation and structural characterization of poly-mannose synthesized by phosphoric acid catalyzation under microwave irradiation
This paper, written by researchers from Jiangnan University and others, discusses Preparation and structural characterization of poly-mannose synthesized by phosphoric acid catalyzation under microwave irradiation. The paper is published in an important journal < Carbohydrate Polymers >. IF:5.158
In recent years, the research work of microwave chemical instrument used in the synthesis of materials has become a hot direction of scientific research, which has been paid great attention to by many scholars!
Poly-mannose with molecular weight of 2.457 kDa was synthesized using d-mannose as substrate andphosphoric acid as catalyst under the condition of microwave irradiation for the first time. The optimumreaction conditions were microwave output power of 900 W, temperature 115◦C, proton concentration2.5 mol/L, and microwave irradiation time 5 min. The actual maximum yield was 91.46%. After purified bySepherdex G-25 column chromatography, the structural features of poly-mannose were investigated byhigh-performance anion-exchange chromatography (HPAEC), high-performance gel-permeation chro-matography (HPGPC), infrared (IR) spectroscopy, methylation analysis and NMR spectroscopy analysis(1H,13C, COSY, TOCSY, HMQC, and HMBC). HPAEC analysis showed that the composition of syntheticpolysaccharides was d-mannose, its purity was demonstrated by HPGPC as a single symmetrical sharppeak, and additionally IR spectra demonstrated the polymerization of d-mannose. Methylation anal-ysis and NMR spectroscopy revealed that the backbone of poly-mannose consisting of (1 → 3)-linked _-d-Manp, (1 → 3)-linked _-d-Manp, and (1 → 6)-linked _-d-Manp residues, and the main chain werebranched at the O-2, O-3, O-4, O-6 position.

Fig.1/4↑
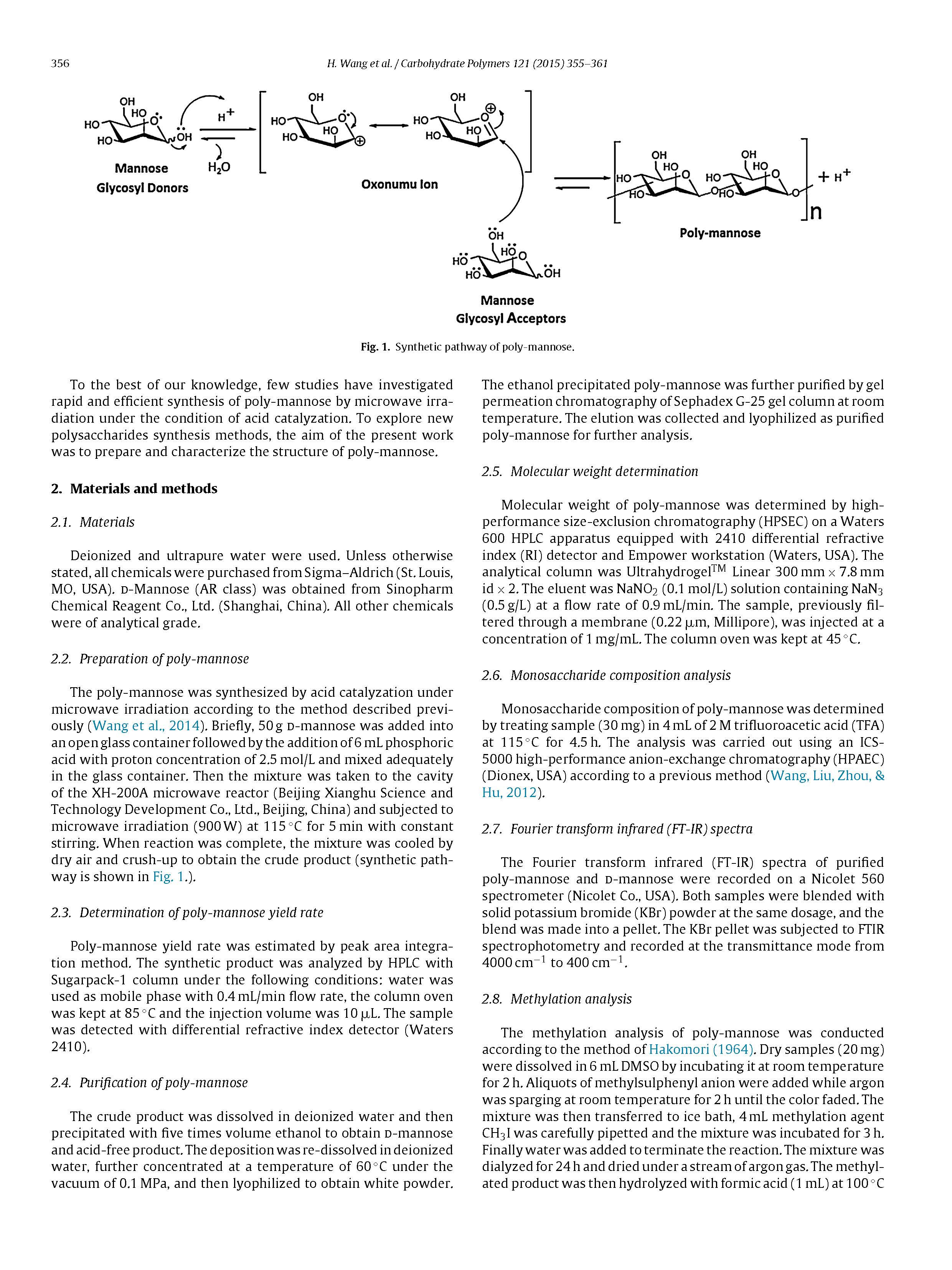
Fig.2/4↑
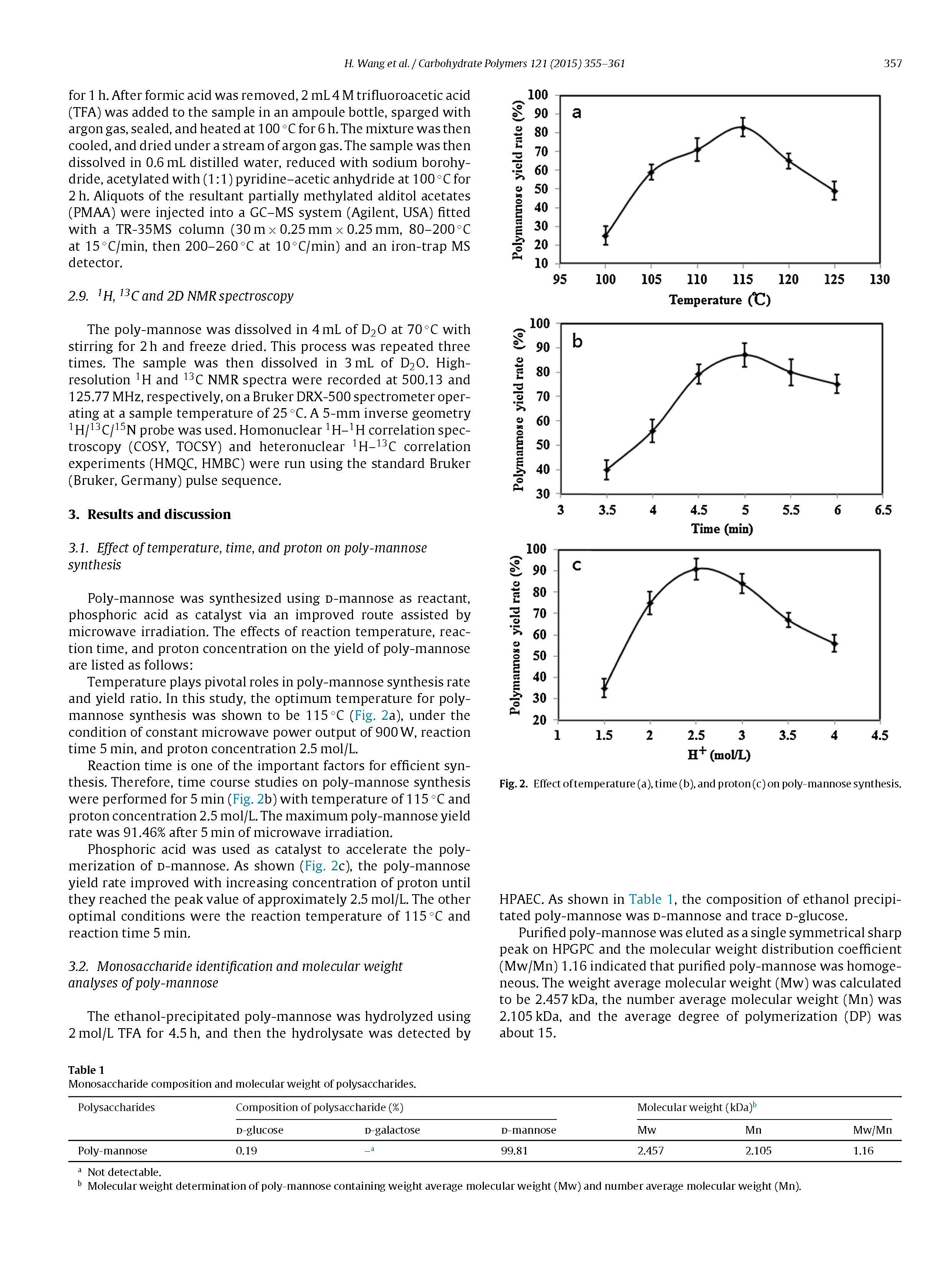
Fig.3/4↑

Fig.4/4↑
In this study, poly-mannose was synthesized using phosphoricacid as catalyst and microwave irradiation as a principal energysource for the first time. The optimum conditions were microwaveoutput power 900 W, reaction temperature 115◦C, proton concen-tration 2.5 mol/L, and microwave irradiation time 5 min. The actualmaximum yield was 91.46%.Additionally the synthesized product was purified and charac-terized by HPAEC, HPGPC, FT-IR spectrum, methylation analysis,and NMR spectroscopy. HPAEC and HPGPC analysis showed that themonosaccharide composition of synthetic polysaccharides was d-mannose and the average degree of polymerization (DP) was about15. FI-IR spectrum indicated the polymerization of d-mannosecatalyzed by acid under the condition of microwave irradiation.Methylation analysis and NMR spectroscopy results suggestedthat both _- and _-anomeric configurations are existing in poly-mannose, the backbone consisting of (1 → 3)-linked _-d-Manp,(1 → 3)-linked _-d-Manp, and (1 → 6)-linked _-d-Manp residues,and the main chain were branched at the O-2, O-3, O-4, O-6 posi-tion.
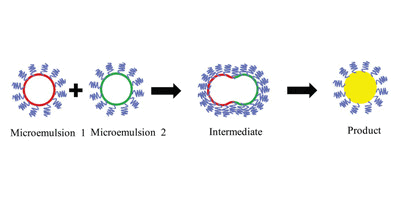
The poly-mannose was synthesized by acid catalyzation undermicrowave irradiation according to the method described previ-ously (Wang et al., 2014). Briefly, 50 g d-mannose was added intoan open glass container followed by the addition of 6 mL phosphoricacid with proton concentration of 2.5 mol/L and mixed adequatelyin the glass container. Then the mixture was taken to the cavityof the XH-200A microwave reactor (Beijing Xianghu Science andTechnology Development Co., Ltd., Beijing, China) and subjected tomicrowave irradiation (900 W) at 115◦C for 5 min with constantstirring. When reaction was complete, the mixture was cooled bydry air and crush-up to obtain the crude product (synthetic path-way is shown in Fig. 1.).








































 京ICP备15050585号
京ICP备15050585号

