- 路 Microwave
- 路 Atmospheric Pressure Microwave 路 Pressure Microwave 路 Parallel Microwave
- 路 Ultrasonic 路Low Temperature Ultrasound
- 路 Ultraviolet Light
- 路 Microwave Heating 路 Atmospheric Pressure Synthesis 路 Atmospheric Pressure Catalysis 路 Atmospheric Pressure Extraction
- 路 Sample Preparation 路 Microwave Digestion
- 路 Soil Digestion 路 High Pressure Synthesis
- 路 Solid Phase Synthesis
- 路 Organic Synthesis
- 路 Ionic Liquid Synthesis
- 路 Degradation Of Natural Organic Matter
- 路 Natural Product Extraction / Purification
河北祥鹄科学仪器有限公司
86 Activation of peroxymonosulfate by microwave irradiation for degradation of organic contaminants
This paper, written by researchers from Beijing Normal University and others, discusses Activation of peroxymonosulfate by microwave irradiation for degradation of organic contaminants. The paper is published in an important journal < Chemical Engineering Journal >. IF:6.735.
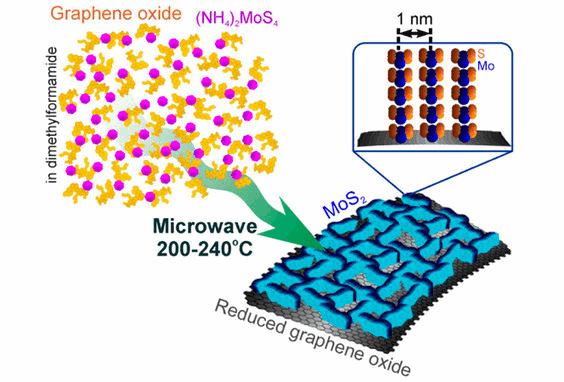
In recent years, the research work of microwave chemical instrument used in the synthesis of materials has become a hot direction of scientific research, which has been paid great attention to by many scholars!
In this study, peroxymonosulfate (PMS) activation was successfully achieved by microwave (MW) irradiation directly and subsequently applied for the degradation of bisphenol A (BPA, an endocrine disrupting chemical frequently detected in the environment), especially at temperatures above 60 _C. The experiment results showed that a higher reaction temperature, MW power level, initial PMS dose, and initial solution pH had positive effects on the degradation efficiency of BPA. The degradation efficiency of BPA was slightly enhanced in real water compared to that in ultrapure water. The result of radical scavenger experiments indicated that both sulfate radical and hydroxyl radical were the dominant reactive oxygen species. Based on the results of high performance liquid chromatography and gas chromatography-mass spectrometry, several transformation pathways, including b-scission, hydroxylation, dehydration, oxidative skeletal rearrangement, and ring opening, were proposed. The complete degradation of several typical organic contaminants was also achieved using the MW/PMS process. This work would broaden the selection of PMS activation methods and provide an option for wastewater treatment.
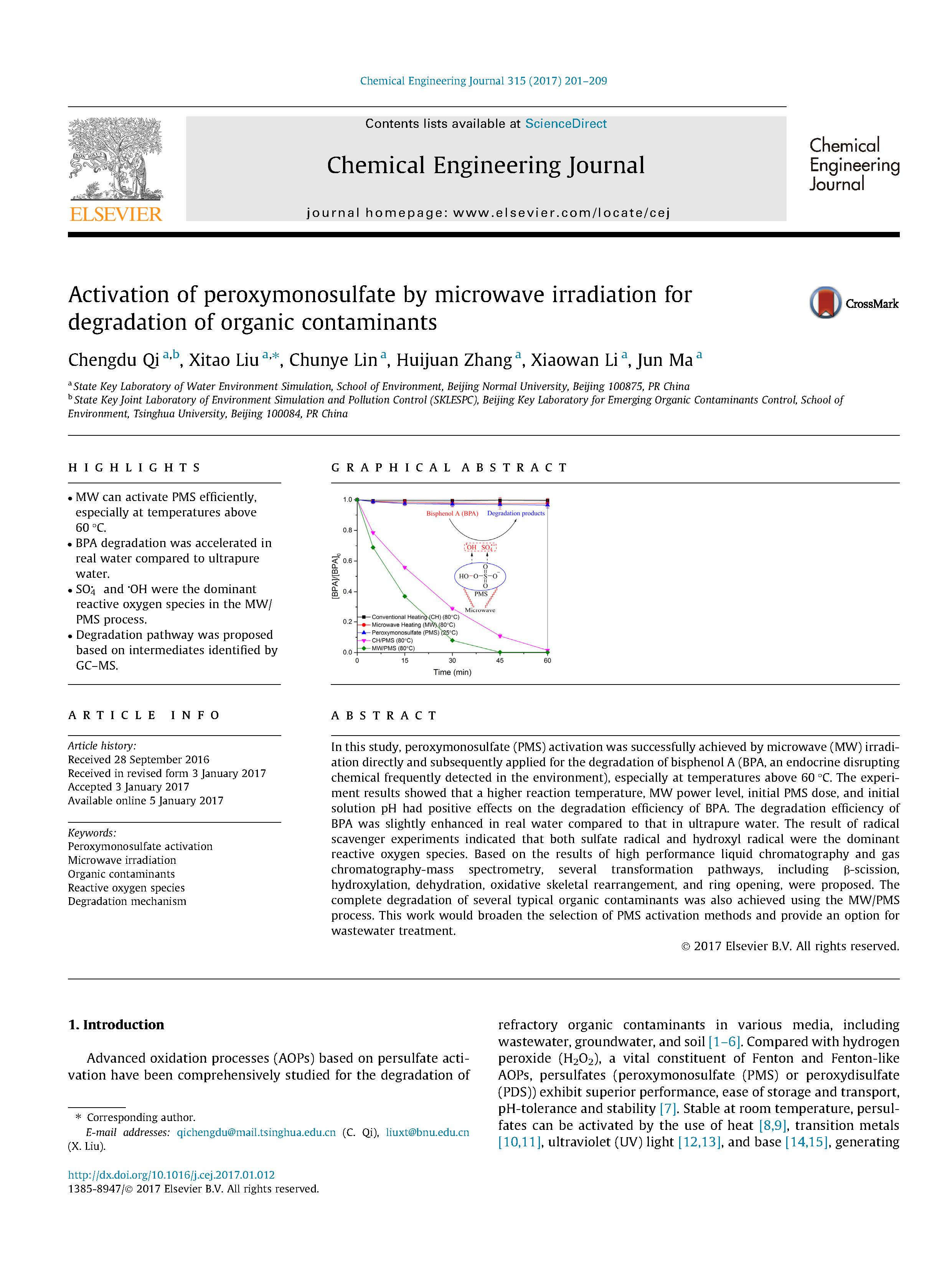
Fig.1/4↑

Fig.2/4↑
Fig.3/4↑

Fig.4/4↑
In summary, MWirradiation was proven to achieve PMS activation directly for the degradation of BPA, especially at temperatures above 60 _C. Both the degradation efficiency and pseudofirst- order degradation rate constant of BPA increased with the increasing of reaction temperature, MW power, initial PMS concentration, and initial solution pH. The degradation efficiency of BPA was substantially enhanced in real water (BW and TW). The sulfate radical and hydroxyl radical were the dominant reactive oxygen species generated in the MW/PMS process. Furthermore, the degradation intermediates of BPA were identified and several transformation pathways were proposed. A certain degree of TOC removal and a high consumption of PMS were observed in the MW/PMS process. In addition, the complete degradation of several typical organic contaminants was achieved in the MW/PMS process. Therefore, the MW/PMS process provides an option and would be a promising technology for wastewater treatment.
A stock solution of BPA (100 mg_L_1) was prepared with ultrapure water prior to each batch experiment and stirred overnight at room temperature until complete BPA dissolution. PMS stock solutions (typically 0.5 M) were prepared before use. MW heating degradation experiments were carried out in a 250-mL threenecked round-bottomed flask equipped with a reflux condenser. The temperature of the reaction mixture was measured by an immersed platinum resistance thermometer. The flask was placed on a commercial MW reactor (XH-100A, 100–1000 W, Beijing Xianghu Science and Technology Development Co., Ltd) that controlled all of the experimental conditions, including the reaction temperature, MW power, stirring speed, and reaction time. Two hundred and fifty milliliters of the BPA solution (typically 87.6 lM, 20 mg_L_1) was heated in the XH-100A MW reactor with an appropriate MW power level (typically 500 W) to reach and maintain the preset temperature (typically 80 _C). The reaction was initiated once 5 mL of the PMS stock solution (typically 0.5 M) was added, except in the studies investigating the effect of the MW power. In this case, PMS and BPA were premixed and then the reaction was initiated onceMWreactor is heated at different MW power levels to a preset temperature (if can reach). Typically, the degradation experiments were conducted at the natural pH of the reaction mixture (2.45), except for the studies on the effect of the initial solution pH. In such cases, the initial solution pH was adjusted to the desired values using appropriate volumes of 0.1 M NaOH or H2SO4. During treatment, rapid stirring (approximately 500 r_min_1) with a magnet ensured a completely mixed solution state. The experiments for the degradation of AO7, MB, ATZ, IBP, and SMX were performed under identical conditions. The temperature effects with conventional heating were also investigated and compared by maintaining the temperature of the BPA solution at 40, 50, 60, 70, and 80 _C using a thermostatically controlled water bath (THZ-82A, China Jiangsu Jingtan Ronghua Instrumental Factory). At given reaction time intervals (0, 5, 15, 30, 45, and 60 min), approximately 5 mL samples were withdrawn and chilled in an ice bath for 30 min to stop the reaction, after which they were kept in a 4 _C refrigerator until further treatment and analysis. All of the experiments were carried out in duplicate.








































 京ICP备15050585号
京ICP备15050585号

