- 路 Microwave
- 路 Atmospheric Pressure Microwave 路 Pressure Microwave 路 Parallel Microwave
- 路 Ultrasonic 路Low Temperature Ultrasound
- 路 Ultraviolet Light
- 路 Microwave Heating 路 Atmospheric Pressure Synthesis 路 Atmospheric Pressure Catalysis 路 Atmospheric Pressure Extraction
- 路 Sample Preparation 路 Microwave Digestion
- 路 Soil Digestion 路 High Pressure Synthesis
- 路 Solid Phase Synthesis
- 路 Organic Synthesis
- 路 Ionic Liquid Synthesis
- 路 Degradation Of Natural Organic Matter
- 路 Natural Product Extraction / Purification
河北祥鹄科学仪器有限公司
160 Construction of amorphous TiO2/BiOBr heterojunctions via facetscoupling for enhanced photocatalytic activity
This paper, written by researchers from Hebei University of Science and Technology and others, discusses Construction of amorphous TiO2/BiOBr heterojunctions via facetscoupling for enhanced photocatalytic activity. The paper is published in an important journal < Journal of Hazardous Materials >.IF:6.434.
In recent years, the research work of microwave chemical instrument used in the synthesis of materials has become a hot direction of scientific research, which has been paid great attention to by many scholars!
Facets coupled BiOBr with amorphous TiO2composite photocatalysts are synthesized via an in situ directgrowth approach under microwave irradiation. XRD, SEM and HRTEM characterizations indicate that theheterointerface between BiOBr and amorphous TiO2occurs mainly on the {001} facets of BiOBr. BETand TEM verify that the heterojunctions possess higher specific surface areas and smaller amorphousTiO2particle size than bare BiOBr and amorphous TiO2, exhibiting the inhibition function of BiOBr onthe growth of TiO2particles. XPS verifies the interaction between the two components. The degradationof methyl orange (MO) and phenol are used as the objective reaction to evaluate the photocatalyticactivity of the as-prepared samples. The reaction rate constant of 15% TiO2/BiOBr composite is 3.4 timesgreater than that of pure BiOBr, which is attributed to its higher surface area, and efficient separation ofphoto-generated electron–hole pairs between BiOBr and amorphous TiO2.
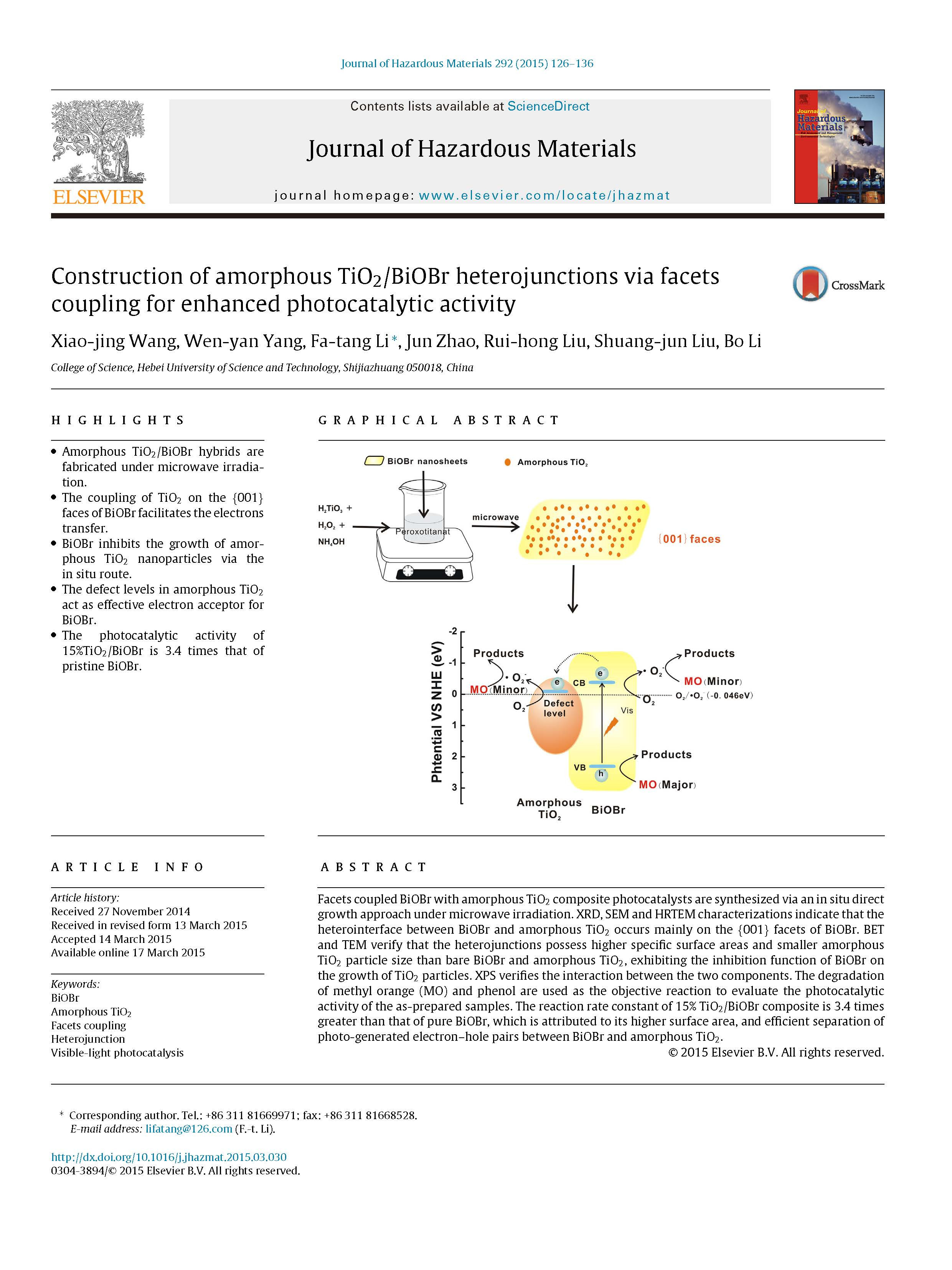
Fig.1/4↑

Fig.2/4↑

Fig.3/4↑

Fig.4/4↑
Novel composites comprising amorphous TiO2coupled withthe {001} facets dominated BiOBr were synthesized via an in situgrowth route. The suitable preparation method led to highly dis-persed amorphous TiO2nanoparticles decorated on the surfaceof BiOBr flakes. Due to the uniform distribution of heterointer-faces between the {001} facets of BiOBr and amorphous TiO2and the defect levels in amorphous TiO2could act as the effec-tive electron acceptor for BiOBr, the separation of photo-generatedelectron–hole pairs in BiOBr improved obviously and results in aneffective enhances of photocatalytic activity. The photocatalyticactivity of 15%TiO2/BiOBr is 3.4 times that of pristine BiOBr. Thisresearch can provide a new insight into broadening the applicationsof TiO2and developing highly efficient BiOBr-based photocatalysts.
The amorphous TiO2was formed by metatitanic acid (H2TiO3). First, a certain amount of H2TiO3was added into a mix-ture of purified water, H2O2(30%) and NH3·H2O (28%), whichwas then placed in an ice-water bath. After stirring for 30 min, a homogeneous pale yellow-green peroxotitanate solution wasobtained. At the same time, a certain amount of BiOBr was addedinto 25 mL of distilled water, and then 30 min of sonication wasperformed to completely disperse the BiOBr powder. The peroxoti-tanate solution was then mixed with the BiOBr suspension. Afterbeing stirred for 4 h, the mixture was transferred into a flask andmicrowave-irradiated for 1 h. For the reaction process occurringin the microwave oven (XH-100B, Beijing Xianghu Co., Ltd), thepower and temperature were set to 500 W and 80◦C, respectively. Finally, the samples were separated by vacuum filtration and driedin an oven at 60◦C. Pure amorphous TiO2was synthesized underthe same conditions without adding BiOBr. The final contents ofTiO2in TiO2/BiOBr composites were determined by the additionproportion of metatitanic acid and BiOBr powders. The obtainedsamples were referred to as X % TiO2/BiOBr, X% is the contents ofTiO2. In order to study the role of anatase TiO2in the composite,the best sample was calcined under 400◦C and referred to as X% TiO2/BiOBr-400. X% TiO2/BiOBr (M) was obtained by adding ofBiOBr microspheres replace of BiOBr nanoflakes.








































 京ICP备15050585号
京ICP备15050585号

